This site is intended for US audiences only
EVOMELA®
Stable by design.
Indications and Usage
EVOMELA is an alkylating drug indicated for:
- Use as a high-dose conditioning treatment prior to hematopoietic progenitor (stem) cell transplantation in patients with multiple myeloma.
- The palliative treatment of patients with multiple myeloma for whom oral therapy is not appropriate.
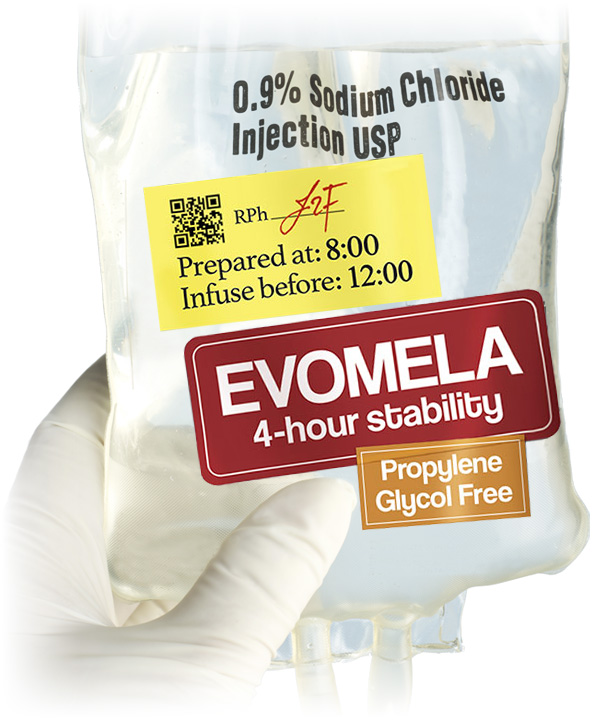
Reconstitution of EVOMELA
Play the video below to learn more about the EVOMELA reconstitution process:
- EVOMELA has a propylene glycol-free formulation1
- Reconstituted product stable for 24 hours at refrigerated temperature (5°C/ 41°F) without precipitation1
- Reconstituted product stable for 1 hour at room temperature1
- Admixture solution is stable for 4 hours at room temperature in addition to the 1 hour following reconstitution1
- Administration recommendations:
- Infuse over 30 minutes for conditioning treatment
- Infuse over 15-20 minutes for palliative treatment
- EVOMELA was FDA approved based, in part, on its bioequivalence to Alkeran via the 505(b)(2) New Drug Application regulatory pathway.1-3
- EVOMELA has a PK profile comparable to conventional IV melphalan2
- EVOMELA has a proven safety and efficacy profile for myeloablative conditioning in multiple myeloma patients undergoing ASCT.1,4
- EVOMELA is also indicated for palliative treatment for patients with multiple myeloma for whom oral therapy is not appropriate.
- Severe bone marrow suppression with resulting infection or bleeding may occur. Controlled trials comparing intravenous (IV) melphalan to oral melphalan have shown more myelosuppression with the IV formulation. Monitor hematologic laboratory parameters.
- Hypersensitivity reactions, including anaphylaxis, have occurred in approximately 2% of patients who received the IV formulation of melphalan. Discontinue treatment with EVOMELA for serious hypersensitivity reactions.
- Melphalan produces chromosomal aberrations in vitro and in vivo. EVOMELA should be considered potentially leukemogenic in humans.
References
-
EVOMELA [prescribing information]. East Windsor, NJ: Acrotech Biopharma, LLC.
-
Aljitawi OS, Ganguly S, Abhyankar SH, et al. Phase IIa cross-over study of propylene glycol-free melphalan (LGD-353) and Alkeran in multiple myeloma autologous transplantation. Bone Marrow Transplant. 2014;49(8):1042-1045.
-
Melphalan Hydrochloride for Injection [prescribing information]. Mylan Institutional LLC; August 2012.
-
Hari P, Aljitawi OS, Arce-Lara C, et al. A phase IIb, multicenter, open-label, safety, and efficacy study of high-dose, propylene glycol-free melphalan hydrochloride for injection (EVOMELA) for myeloablative conditioning in multiple myeloma patients undergoing autologous transplantation. Biol Blood Marrow Transplant. 2015;21(12):2100-2105.

