Evomela® Stability
Evomela®—Stable by design
Healthcare professionals involved with melphalan preparation and administration can have access to a formulation with 4-hour admixture stability. Evomela® offers the therapeutic benefits you expect, with stability that allows for scheduling flexibility.1

- Bioequivalent to conventional IV melphalan2
- The only IV melphalan product that is FDA approved for use as a high-dose conditioning treatment in patients with multiple myeloma undergoing autologous stem cell transplantation1,4

- 24-hour stability after reconstitution when refrigerated at 5°C (41°F)
- 1-hour stability after reconstitution at room temperature1
- Propylene glycol–free formulation1-3

- 4-hour admixture stability opens a 4-hour window of time to complete an infusion1
- Administration recommendation: infuse over 30 minutes for conditioning treatment1,3
Selected Important Safety Information
Contraindications
- History of serious allergic reaction to melphalan.
Warnings and Precautions
Secondary Malignancies:
- Melphalan has been shown to cause chromatid or chromosome damage in humans. Secondary malignancies such as myeloproliferative syndrome or acute leukemia have been reported in multiple myeloma patients treated with melphalan-containing chemotherapy regimens. The potential benefit of Evomela® therapy must be considered against the possible risk of the induction of a secondary malignancy.
Embryo-Fetal Toxicity:
- Based on its mechanism of action, Evomela® can cause fetal harm when administered to a pregnant woman. Melphalan is genotoxic, targets actively dividing cells, and was embryolethal and teratogenic in rats. Advise pregnant women of the potential risk to a fetus. Advise females of reproductive potential to use effective contraception during treatment with Evomela® and for 6 months after the last dose. Advise males with female partners of reproductive potential to use effective contraception during treatment with Evomela® and for 3 months after the last dose.
Infertility:
- Melphalan-based chemotherapy regimens have been reported to cause suppression of ovarian function in premenopausal women, resulting in persistent amenorrhea in approximately 9% of patients. Reversible or irreversible testicular suppression has also been reported.
Evomela®—4‐hour admixture stability1
As a reconstituted drug product:
- Stable for 24 hours at 5°C (41°F), without precipitation1
- Stable for 1 hour at room temperature1
As an admixture:
- Stable for 4 hours at room temperature after reconstitution1
Preparation and Administration1
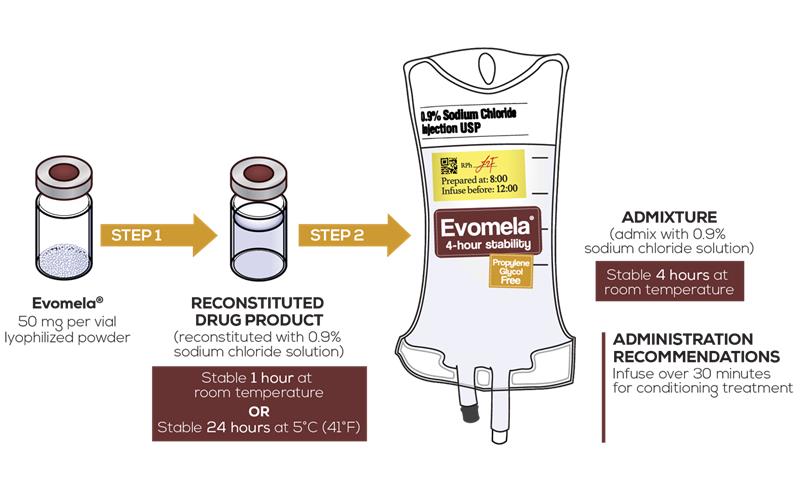
Important Preparation and Administration Information
- Evomela® is a hazardous drug. Follow applicable special handling and disposal procedures.1
- Evomela® is light sensitive. Retain in original carton until use.1
- Do not mix Evomela® with other melphalan hydrochloride for injection drug products.1
- Evomela® may cause local tissue damage should extravasation occur. Do not administer by direct injection into a peripheral vein. Administer Evomela® by injecting slowly into a fast-running IV infusion via a central venous access line.1
- Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.1
Evomela®—A propylene glycol–free formulation1-3
Evomela® is formulated with a modified cyclodextrin, betadex sulfobutyl ether sodium.1,2,5
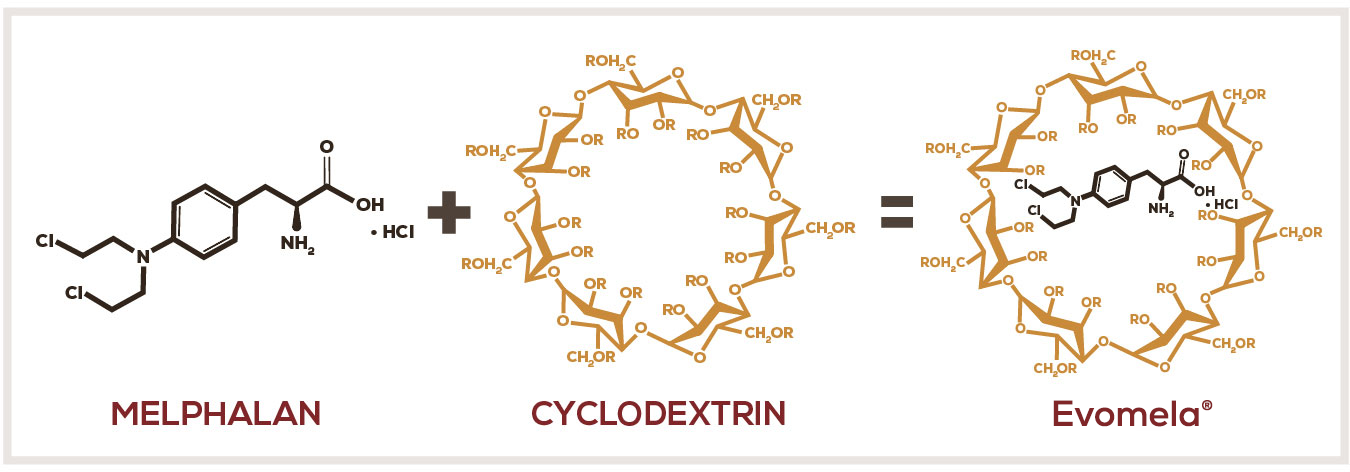
- The modified cyclodextrin forms reversible inclusion complexes with melphalan that improve solubility and stability in aqueous solutions5
- Once in the bloodstream, the cyclodextrin rapidly dissociates from melphalan due to dilution by blood5
Selected Important Safety Information
Warnings and Precautions
Gastrointestinal Toxicity:
- For patients receiving Evomela® as part of a conditioning regimen, nausea, vomiting, mucositis, and diarrhea may occur in over 50% of patients. Use prophylactic antiemetic medication. Provide supportive care for nausea, vomiting, diarrhea, and mucositis. The frequency of grade 3/4 mucositis in clinical studies was 13%. Provide nutritional support and analgesics for patients with severe mucositis.
References
-
Evomela® Prescribing Information. Acrotech Biopharma Inc.
-
Aljitawi OS, Ganguly S, Abhyankar SH, et al. Phase IIa cross-over study of propylene glycol-free melphalan (LGD-353) and alkeran in multiple myeloma autologous transplantation. Bone Marrow Transplant. 2014;49(8):1042-1045.
-
Hari P, Aljitawi OS, Arce-Lara C, et al. A phase IIb, multicenter, open-label, safety, and efficacy study of high-dose, propylene glycol–free melphalan hydrochloride for injection (Evomela®) for myeloablative conditioning in multiple myeloma patients undergoing autologous transplantation. Biol Blood Marrow Transplant. 2015;21(12):2100-2105.
-
FDA. Center for Drug Evaluation and Research. Evomela® Drug Application. 2016.
-
Stella VJ, He Q. Cyclodextrins. Toxicol Pathol. 2008;36(1):30-42.



