Evomela® Efficacy & Safety
Evomela®—Efficacy and safety demonstrated in a pivotal clinical study1,2
Evomela® was evaluated for myeloablative conditioning in multiple myeloma patients undergoing autologous stem cell transplantation (ASCT) in a Phase IIb, multicenter, open-label, single-arm, nonrandomized US clinical study (n=61).1,3
Please see pivotal study design here.
Study confirmed the efficacy and acceptable safety profile of Evomela® as a high-dose conditioning regimen for ASCT in patients with multiple myeloma.1,3
Contraindicated in patients with a history of serious allergic reaction to melphalan. Please see the safety profile of Evomela®.1
Proven efficacy
- An overall response rate of 95% at follow-up (Day +90 to Day +100)1
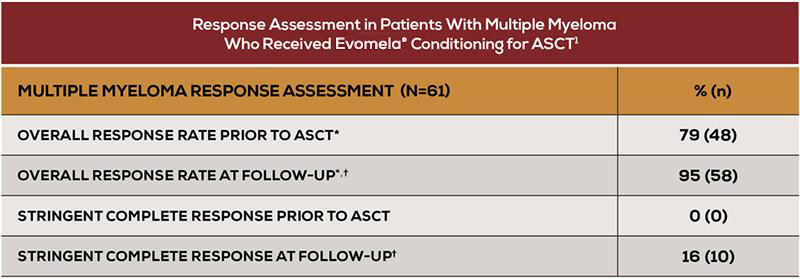
*Overall response rate is categorized as partial response or better.1 † Follow-up = Day +90 to Day +100.1
Myeloablation and engraftment achieved in all patients1
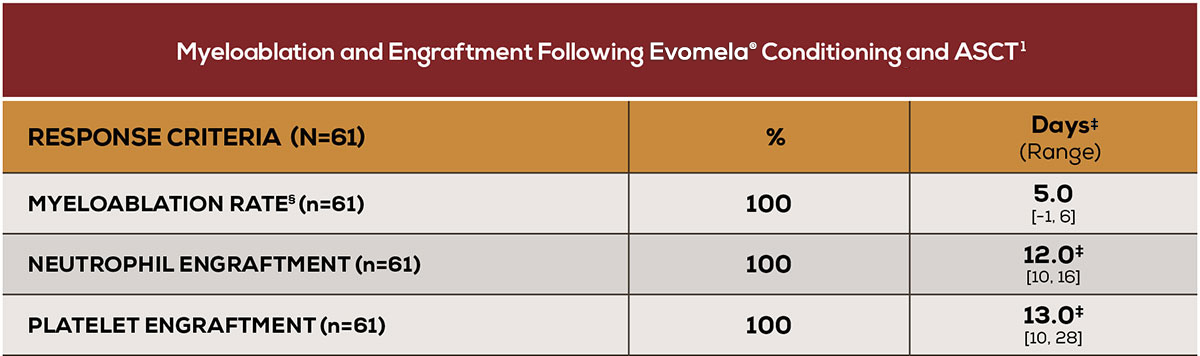
‡Median time to myeloablation or engraftment.1 §Myeloablation was defined as any of the following: absolute neutrophil count (ANC) <500/mm3, absolute lymphocyte count <100/mm3, or platelet count <20,000/mm3. Neutrophil engraftment was defined as ANC >500/mm3 × 3 consecutive daily assessments. Platelet engraftment was defined as untransfused platelet counts >20,000/mm3 × 3 consecutive daily assessments. Nonengraftment was defined as failure to reach an ANC >500/mm3 × 3 consecutive daily assessments by Day 90–100.1
Selected Important Safety Information
Warnings and Precautions
Gastrointestinal Toxicity:
- For patients receiving Evomela® as part of a conditioning regimen, nausea, vomiting, mucositis, and diarrhea may occur in over 50% of patients. Use prophylactic antiemetic medication. Provide supportive care for nausea, vomiting, diarrhea, and mucositis. The frequency of grade 3/4 mucositis in clinical studies was 13%. Provide nutritional support and analgesics for patients with severe mucositis.
References
-
Evomela® Prescribing Information. Acrotech Biopharma Inc.
-
Aljitawi OS, Ganguly S, Abhyankar SH, et al. Phase IIa cross-over study of propylene glycol–free melphalan (LGD-353) and alkeran in multiple myeloma autologous transplantation. Bone Marrow Transplant. 2014;49(8):1042-1045.
-
Hari P, Aljitawi OS, Arce-Lara C, et al. A phase IIb, multicenter, open-label, safety, and efficacy study of high-dose, propylene glycol–free melphalan hydrochloride for injection (Evomela®) for myeloablative conditioning in multiple myeloma patients undergoing autologous transplantation. Biol Blood Marrow Transplant. 2015;21(12):2100-2105.



