EVOMELA Bioequivalence
EVOMELA—Bioequivalent to conventional IV melphalan based on Phase IIa cross-over study1-3
EVOMELA was FDA approved based, in part, on its bioequivalence to Alkeran via the 505(b)(2) New Drug Application regulatory pathway.1-3
EVOMELA has a PK profile comparable to conventional IV melphalan2
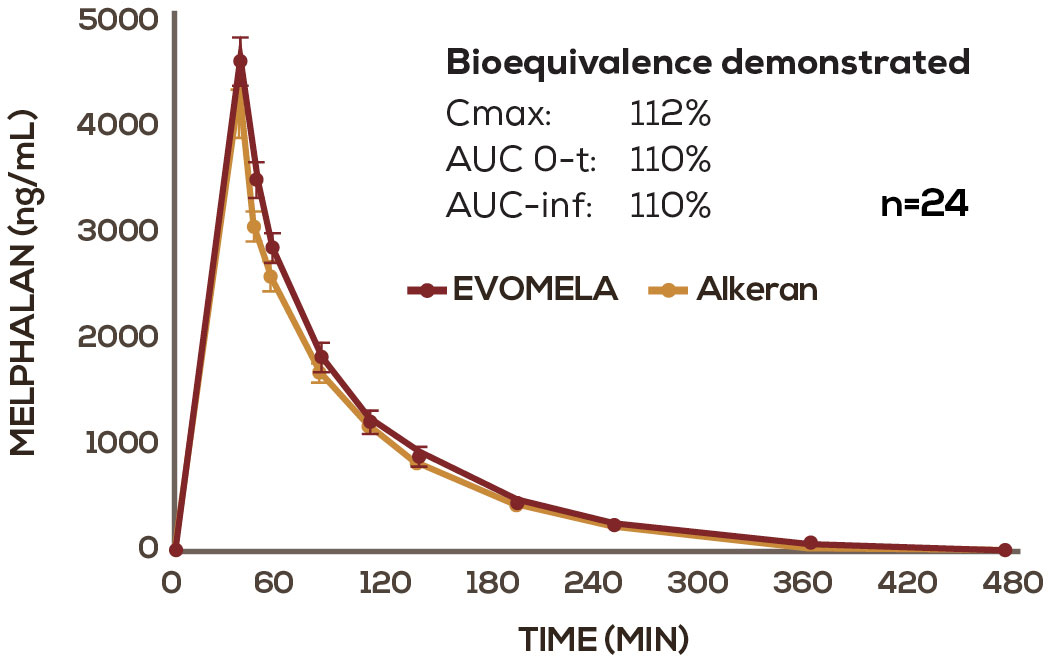
Mean melphalan plasma concentration-time profiles following infusion of EVOMELA and Alkeran. Alkeran (melphalan) is a registered trademark of GlaxoSmithKline, distributed by ApoPharma USA Inc.
Selected Important Safety Information
Warnings and Precautions
Bone Marrow Suppression:
- For patients receiving EVOMELA as part of a conditioning regimen, myeloablation occurs in all patients. Do not begin the conditioning regimen if a stem cell product is not available for rescue. Monitor complete blood counts, provide supportive care for infections, anemia and thrombocytopenia until there is adequate hematopoietic recovery.
- For patients receiving EVOMELA as palliative treatment, if the bone marrow has been compromised by prior irradiation, prior chemotherapy or is recovering from chemotherapy, the risk of severe myelosuppression with EVOMELA is increased. Perform periodic complete blood counts during the course of treatment with EVOMELA. Provide supportive care for infections, bleeding, and symptomatic anemia.
References
-
EVOMELA [prescribing information]. East Windsor, NJ: Acrotech Biopharma, LLC.
-
Aljitawi OS, Ganguly S, Abhyankar SH, et al. Phase IIa cross-over study of propylene glycol-free melphalan (LGD-353) and alkeran in multiple myeloma autologous transplantation. Bone Marrow Transplant. 2014;49(8):1042-1045.
-
Melphalan Hydrochloride for Injection [prescribing information]. Mylan Institutional LLC; August 2012.

