Evomela® Bioequivalence
Evomela®—Bioequivalent to conventional IV melphalan based on Phase IIa cross-over study2
Evomela® was FDA approved based, in part, on its bioequivalence to Alkeran via the 505(b)(2) New Drug Application regulatory pathway.1-4
Evomela® has a PK profile comparable to conventional IV melphalan2
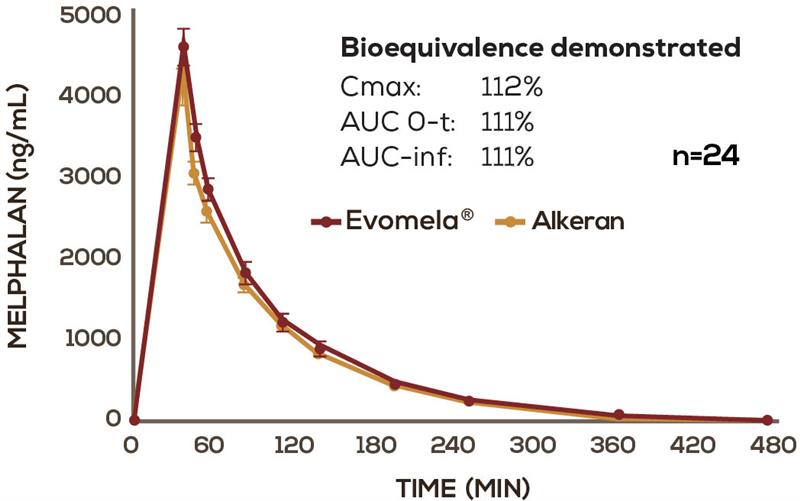
Mean (+/- s.e.m.) melphalan plasma concentration-time profiles following infusion of Evomela® and Alkeran. Alkeran (melphalan) is a registered trademark of GlaxoSmithKline, distributed by ApoPharma USA Inc.
Selected Important Safety Information
Warnings and Precautions
Bone Marrow Suppression:
- For patients receiving Evomela® as part of a conditioning regimen, myeloablation occurs in all patients. Do not begin the conditioning regimen if a stem cell product is not available for rescue. Monitor complete blood counts, provide supportive care for infections, anemia and thrombocytopenia until there is adequate hematopoietic recovery.
References
-
Evomela® Prescribing Information. Acrotech Biopharma Inc.
-
Aljitawi OS, Ganguly S, Abhyankar SH, et al. Phase IIa cross-over study of propylene glycol-free melphalan (LGD-353) and alkeran in multiple myeloma autologous transplantation. Bone Marrow Transplant. 2014;49(8):1042-1045.
-
Hari P. Aijitawi OS. Arce-Lara C. et al. A phase IIb. multicenter. open-label. safety. and efficacy study of high-dose. propylene glycol-free melphalan hydrochloride for injection (Evomela®) for myeloablative conditioning in multiple myeloma patients undergoing autologous transplantation. Biol Blood Marrow Transplant. 2015:21(12):2100-2105.
-
FDA. Center for Drug Evaluation and Research. Evomela® Drug Application. 2016.



