Common Adverse Reactions
Evomela®—Safety profile
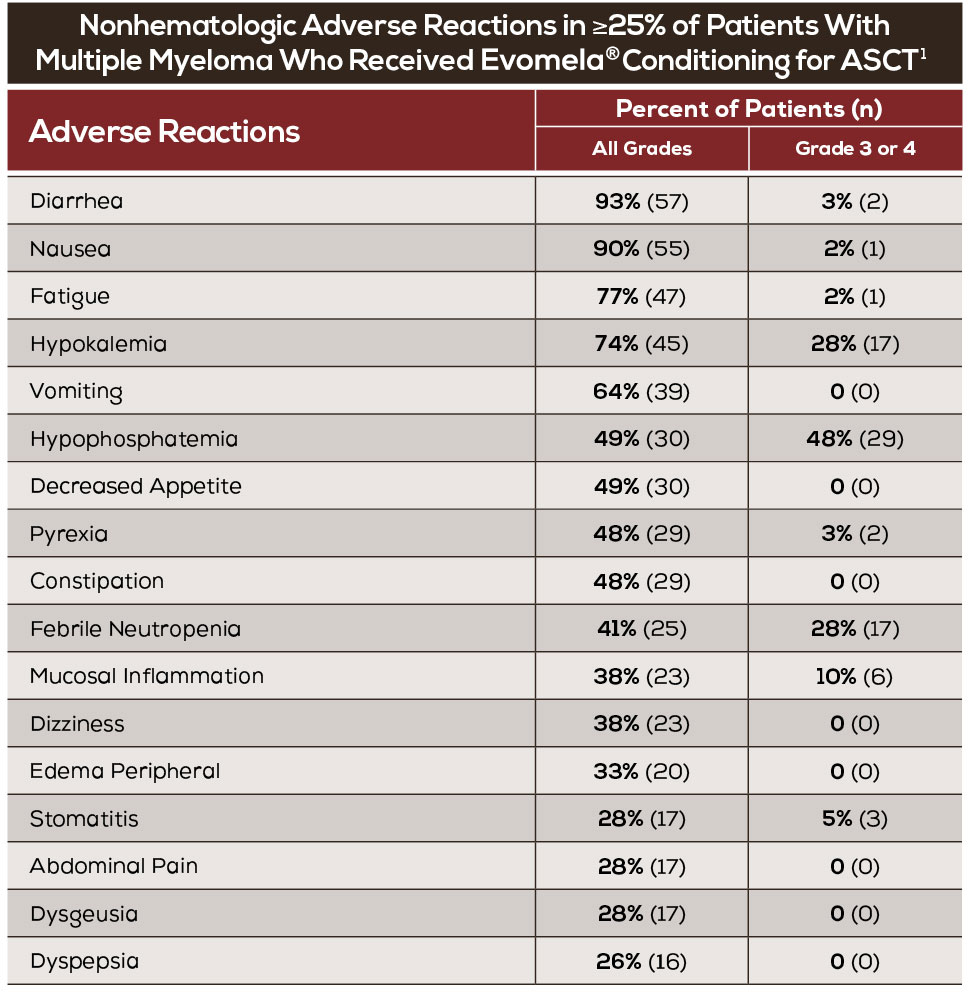
Selected Important Safety Information
Adverse Events
- The most common adverse reactions observed in at least 50% of patients with multiple myeloma treated with Evomela® were neutrophil count decreased, white blood cell count decreased, lymphocyte count decreased, platelet count decreased, diarrhea, nausea, fatigue, hypokalemia, anemia, and vomiting.1
- For myeloablative conditioning in multiple myeloma patients undergoing ASCT, twelve (20%) patients experienced a treatment emergent serious adverse reaction while on study. The most common serious adverse reactions (>1 patient, 1.6%) were pyrexia, hematochezia, febrile neutropenia, and renal failure. Treatment-related serious adverse reactions reported in >1 patient were pyrexia (n=2, 3%), febrile neutropenia (n=2, 3%), and hematochezia (n=2, 3%).1
Reference
-
Evomela® Prescribing Information. Acrotech Biopharma Inc.



