EVOMELA Stability
EVOMELA—Stable by design
Healthcare professionals involved with melphalan preparation and administration can have access to a formulation with 4-hour admixture stability. EVOMELA offers the therapeutic benefits you expect, with stability that allows for scheduling flexibility.1

- Bioequivalent to conventional IV melphalan2
- The only IV melphalan product that is FDA approved for use as a high-dose conditioning treatment in patients with multiple myeloma undergoing autologous stem cell transplantation1,4

- 24-hour stability after reconstitution when refrigerated at 5°C (41°F)
- 1-hour stability after reconstitution at room temperature1
- Propylene glycol–free formulation1-3

- 4-hour admixture stability opens a 4-hour window of time to complete an infusion1
- Administration recommendation: infuse over 30 minutes for conditioning treatment1,3
Selected Important Safety Information
Contraindications
- History of serious allergic reaction to melphalan.
Warnings and Precautions
Secondary Malignancies:
- Secondary malignancies such as myeloproliferative syndrome or acute leukemia have been reported in multiple myeloma patients treated with melphalan-containing chemotherapy regimens. The potential benefit of EVOMELA therapy must be considered against the possible risk of the induction of a secondary malignancy.
Embryo-Fetal Toxicity:
- EVOMELA can cause fetal harm when administered to a pregnant woman. Advise pregnant women of the potential risk to fetus. Advise females of reproductive potential to use effective contraception during treatment with EVOMELA and for 6 months after the last dose. Advise males with female partners of reproductive potential to use effective contraception during treatment with EVOMELA and for 3 months after the last dose.
Infertility:
- Melphalan-based chemotherapy regimens have been reported to cause suppression of ovarian function in premenopausal women, resulting in persistent amenorrhea in approximately 9% of patients. Reversible or irreversible testicular suppression has also been reported.
EVOMELA—4‐hour admixture stability1
As a reconstituted drug product:
- Stable for 24 hours at 5°C (41°F), without precipitation1
- Stable for 1 hour at room temperature1
As an admixture:
- Stable for 4 hours at room temperature after reconstitution1
Preparation and Administration1
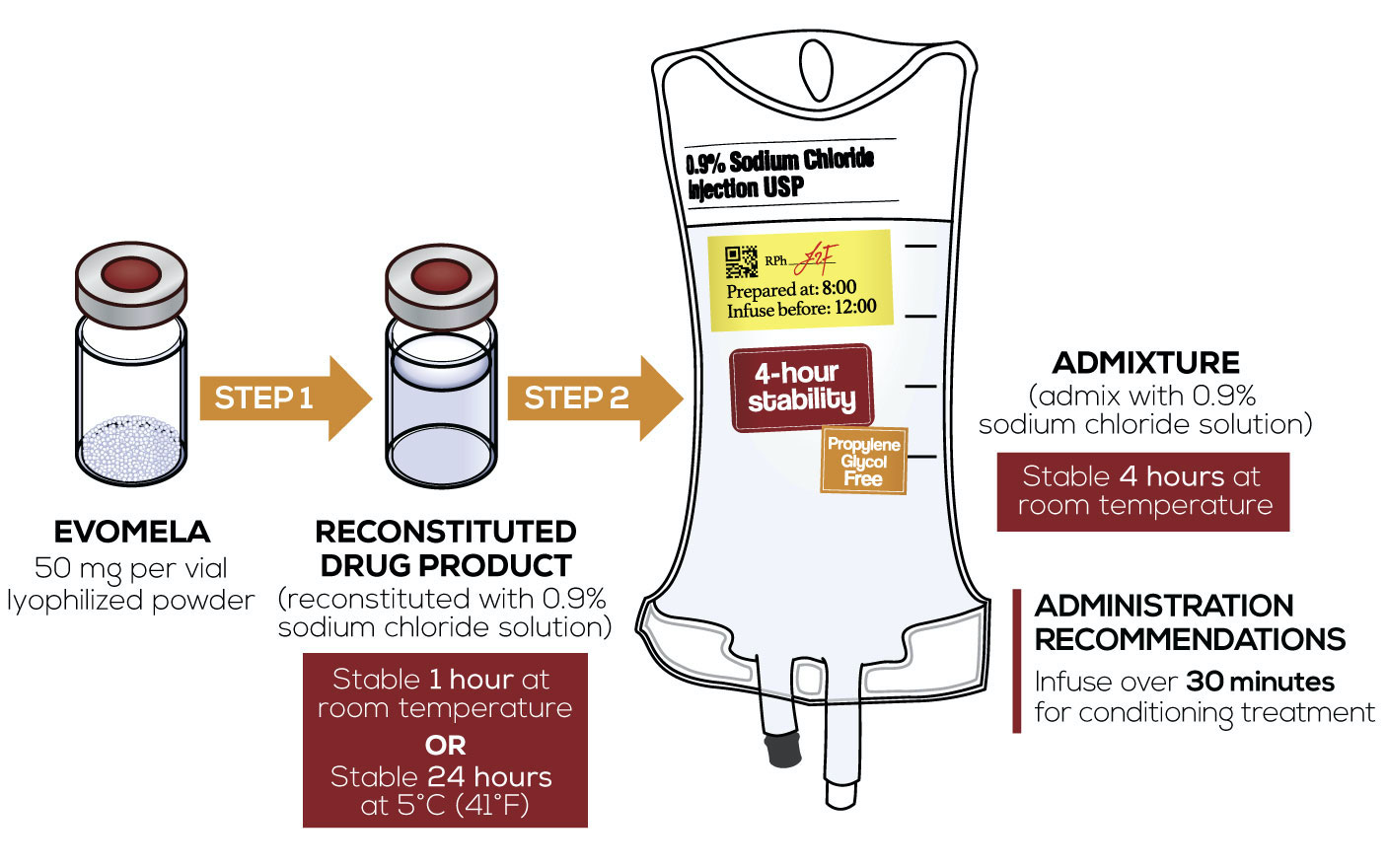
Important Preparation and Administration Information
- EVOMELA is a hazardous drug. Follow applicable special handling and disposal procedures.1
- EVOMELA is light sensitive. Retain in original carton until use.1
- Do not mix EVOMELA with other melphalan hydrochloride for injection drug products.1
- EVOMELA may cause local tissue damage should extravasation occur. Do not administer by direct injection into a peripheral vein. Administer EVOMELA by injecting slowly into a fast-running IV infusion via a central venous access line.1
- Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.1
EVOMELA—A propylene glycol–free formulation1-3
EVOMELA is formulated with a modified cyclodextrin, betadex sulfobutyl ether sodium.1,2,5
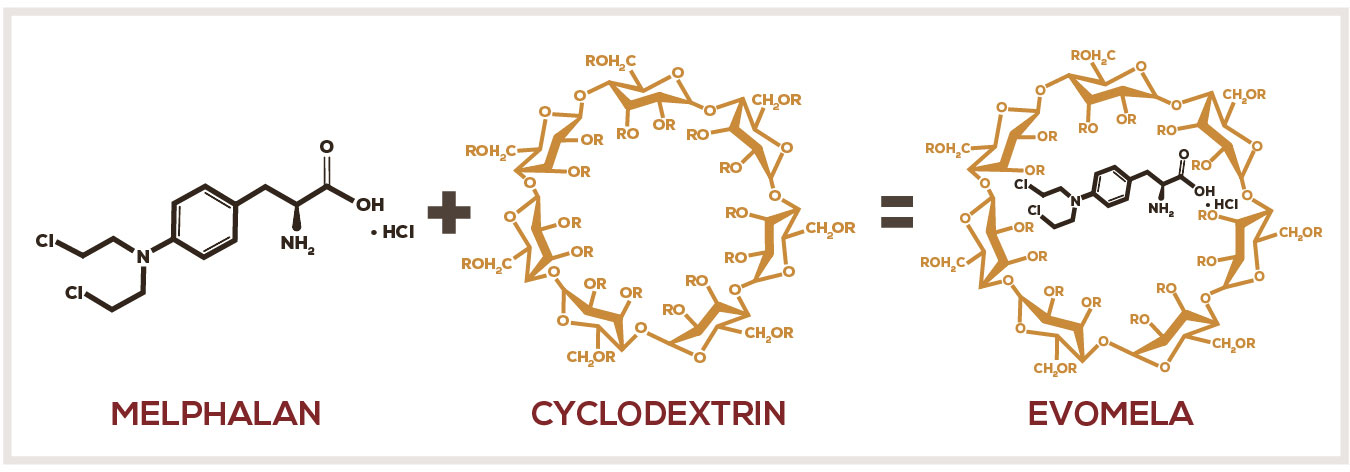
- The modified cyclodextrin forms reversible inclusion complexes with melphalan that improve solubility and stability in aqueous solutions5
- Once in the bloodstream, the cyclodextrin rapidly dissociates from melphalan due to dilution by blood5
Selected Important Safety Information
Warnings and Precautions
Gastrointestinal Toxicity:
- For patients receiving EVOMELA as part of a conditioning regimen, nausea, vomiting, mucositis, and diarrhea may occur in over 50% of patients. Use prophylactic antiemetic medication. Provide supportive care for nausea, vomiting, diarrhea, and mucositis. The frequency of grade 3/4 mucositis in clinical studies was 13%. Provide nutritional support and analgesics for patients with severe mucositis.
References
-
EVOMELA Prescribing Information. Acrotech Biopharma, LLC.
-
Aljitawi OS, Ganguly S, Abhyankar SH, et al. Phase IIa cross-over study of propylene glycol-free melphalan (LGD-353) and alkeran in multiple myeloma autologous transplantation. Bone Marrow Transplant. 2014;49(8):1042-1045.
-
Hari P, Aljitawi OS, Arce-Lara C, et al. A phase IIb, multicenter, open-label, safety, and efficacy study of high-dose, propylene glycol–free melphalan hydrochloride for injection (EVOMELA) for myeloablative conditioning in multiple myeloma patients undergoing autologous transplantation. Biol Blood Marrow Transplant. 2015;21(12):2100-2105.
-
FDA. Center for Drug Evaluation and Research. EVOMELA Drug Application. 2016.
-
Stella VJ, He Q. Cyclodextrins. Toxicol Pathol. 2008;36(1):30-42.

