Study Design
In a pivotal clinical study, EVOMELA demonstrated efficacy and safety1,2
EVOMELA was evaluated for myeloablative conditioning in multiple myeloma patients undergoing autologous stem cell transplantation (ASCT) in an open-label, single-arm, nonrandomized US clinical study (n=61).1,2
Study Design2
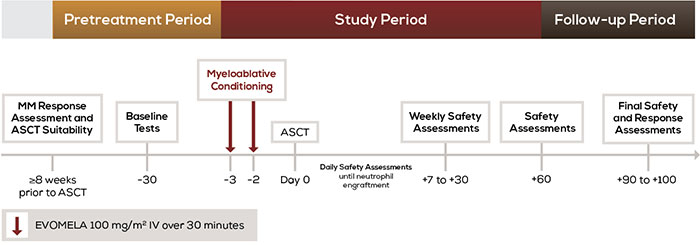
Patients enrolled in the study received 200 mg/m2 of IV melphalan as 2 doses of EVOMELA (100 mg/m2 each) on days –3 and –2 followed by a day of rest before ASCT was performed on Day 0. Patients were evaluated for safety and response through Day +100.2
The efficacy was evaluated by the International Myeloma Working Group response criteria comparing the disease response immediately prior to the ASCT procedure to the disease response assessed 90 to 100 days post-transplant. In addition, successful myeloablation and time to engraftment were evaluated.1,2
Myeloablation and engraftment were evaluated by complete blood cell counts daily until neutrophil and platelet engraftment, and then weekly until Day 30, and at Day 60, and Days 90–100.1,2
Selected Important Safety Information
Warnings and Precautions
Hepatotoxicity:
- Hepatic disorders ranging from abnormal liver function tests to clinical manifestations such as hepatitis and jaundice have been reported after treatment with melphalan. Hepatic veno-occlusive disease has also been reported. Monitor liver chemistries.
References
- EVOMELA Prescribing Information. Acrotech Biopharma, LLC.
- Hari P, Aljitawi OS, Arce-Lara C, et al. A phase IIb, multicenter, open-label, safety, and efficacy study of high-dose, propylene glycol–free melphalan hydrochloride for injection (EVOMELA) for myeloablative conditioning in multiple myeloma patients undergoing autologous transplantation. Biol Blood Marrow Transplant. 2015;21(12):2100-2105.

